
Every first-year chemistry student knows that chemical reaction rates increase with temperature. But not always! In the late '80s at TRIUMF, Don Fleming (TRIUMF/UBC Chemistry) and collaborators demonstrated experimentally a reaction that slowed down with increasing temperature. This seemingly counterintuitive result did not mean that chemistry textbooks needed rewriting but it did demand a detailed theoretical study. Recently published in a prestigious chemistry journal, that work yielded something new and fundamental which ultimately will find its way into next-generation textbooks.
Understanding reaction rates is fundamental to chemistry. They often involve complicated multi-step mechanisms depending on the atomic masses and the chemical bonds between them. The mass dependency can be probed using isotopes (“kinetic isotope effects”), which are atoms with the same number of electrons but different nuclear masses – e.g., the three natural hydrogen isotopes (hydrogen, mass 1amu; deuterium, 2amu; and tritium, 3amu). However, the factor of 3 in mass between the heaviest and lightest hydrogen atoms provides only a limited test of reaction-rate theory. Recently, muon scientists at TRIUMF’s Centre for Molecular and Materials Science (CMMS) extended this mass range to a factor of 36, allowing experimentalists to apply the most stringent tests of rate theory by experiment.
At the CMMS (and elsewhere), Muonium (Mu = μ+e-, 0.114amu), an atom with a positive muon as its nucleus, has long been used as the lightest hydrogen isotope. Recently, muon scientists have introduced as well “superheavy hydrogen” (4Heμ, or simply 4H, 4.11amu), a muonic helium atom where one orbital electron is replaced by a negative muon orbiting so close to the nucleus that it fully screens, or negates, one proton charge, resulting in the heaviest hydrogen-like atom. In 2011 Fleming and coworkers studied the isotopic variants of the fundamental H+H2 reaction through comparisons of experiment with fully-rigorous quantum calculations to demonstrate convincingly that Mu and 4H indeed behave as H-atom isotopes . Fleming and later collaborators then utilized these lightest and heaviest H-atom variants in new work that revealed something uniquely important - the existence of a new type of chemical bond.
Back in 1989 at TRIUMF, Fleming and coworkers observed a reaction between muonium and bromine (Mu + Br2) that slowed down with increasing temperature. They speculated that a transitional state in the reaction could be either a conventional van der Waals-type bond, or the Mu atom could vibrate rapidly between the two Br atoms (like a marble between bowling balls), reducing the overall system energy and giving rise to a vibrational bond. The latter bond had been qualitatively predicted years earlier but never accurately calculated nor experimentally verified.
That changed in 2012 when Fleming and collaborators found one missing piece of the puzzle in a new experiment at the Rutherford Appleton Laboratory in England that clearly established the formation of an intermediate muoniated free-radical complex. However, it was not clear whether it was a Mu-Br2 van der Waals bond or a Br-Mu-Br vibrational bond.
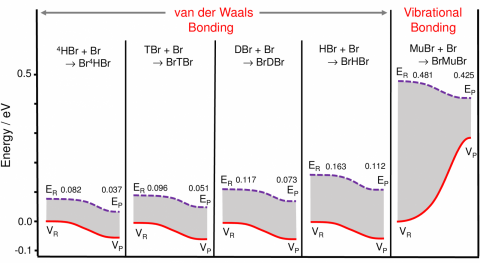
In a prime example of the synergism between theory and experiment, collaborating theoretical chemists were spurred on to undertake the first-ever reliable, fully quantum-mechanical treatment of BrLBr bound states (where L=Mu, H, D, T, 4H). Their results yielded the last puzzle piece when they showed that only Br+MuBr → [Br-Mu-Br] was stable at the barrier maximum of the inter-atomic potential, thus demonstrating vibrational bonding for the first time. This is a purely quantum-mechanical phenomenon in which the high zero-point energy of the MuBr isotopic bond places it above the total ground-state energy of the BrMuBr transition state. In contrast, all the heavier BrLBr “isotopomers” are stabilized only at van der Waals minima far removed from the barrier maximum.
This truly ground-breaking result will open up new avenues of research, and has received much-deserved media attention e.g. in Nature and Scientific American. Fleming and his coworkers predict that this phenomenon will occur in other reactions involving light and heavy atoms. The upshot for undergraduates is that their chemistry textbooks now will have to be changed to include vibrational bonding in the list of known chemical bonds.
You could say that with a Fleming involved, this ‘triumf’ of muon science has shaken, not stirred, historical concepts of chemical bonding.
Figure caption:
Energetics for the conventional van der Waals bonding of the heavy isotopomers of the Br(L)Br system, from the heaviest Br(4H)Br to Br(H)Br (four left panels) contrasted with that for vibrational bonding in Br(Mu)Br, formed with muonium Mu (right panel). The values for the potential energy, VR and VP, and the total ground state energies, ER and EP, for the reactants LBr + Br and products Br(L)Br are shown. Note that only BrMuBr shows a dramatic increase in potential energy needed to overcome the potential barrier, but which is over-compensated by a dramatic decrease in zero-point energy at the saddle point, due to the very light muon mass, providing a net gain in energy for this bound-state system that is stabilized at the maximum of the potential surface.
