[Editor's Note: See the exciting YouTube video about this work!]
Chemical reactions play an important if not crucial role in the processes of life, but the complex dance that Nature choreographs is often difficult to break down into its fundamental steps. In a paper published today by Science (Vol. 331 no. 6016 pp. 448-450), researchers have established a new milestone in the detailed understanding of the chemical reaction processes involving the "simplest" component of all—the hydrogen atom. Experimental measurements have been made that are in accord with detailed, first-principles quantum mechanical predictions for how this chemistry actually takes place.
The paper in Science is entitled "Kinetic Isotope Effects for the Reactions of Muonic Helium and Muonium with H2," a synergistic interplay of theory and experiment, from work at the Centre for Molecular and Materials Science at TRIUMF led by Professor Don Fleming from UBC's Department of Chemistry on the one hand, and headed by the theory group at the University of Minnesota on the other, involving as well theoretical chemists at Northwestern University, Pacific Northwest National Laboratory, and Washington State University.
The study of reaction rates in chemistry has a long history, going back to around the end of the 19th century. Even the seemingly simplest of reactions often proceed by complex mechanisms and it is the role of chemical kinetics and dynamics, which this paper is concerned with, to identify and study key elementary steps in Nature's complex chemical dance. Critical to that endeavour is the use of isotopes in the study of "kinetic isotope effects" (KIEs) since isotopes have different nuclear masses but the same chemical interactions. Thus the reaction dynamics, due to the motion of the nuclear masses, can be very different for different isotopes. By studying the role of nuclear masses in the same chemical reactions, experimentalists and theorists can come to agreement on exactly what is happening at the microscopic level.
As one might expect, it is the isotopes of the H atom that reveal the most dramatic KIEs. Traditionally these studies have relied on comparing the reaction rates of Deuterium with Hydrogen, where the masses differ by a factor of two. While Tritium, with a mass of 3 amu would be useful in this regard, it is radioactive and dangerous and is therefore not readily used in these types of studies. Thus it has fallen to nuclear science and particularly to muon science to extend the isotopic mass scale of the H atom.
The article has established for the first time the reaction rate of the heaviest hydrogen atom, the neutral muonic He atom, 4Heu, which has a mass 4x heavier than the H-atom and which is formed by the capture of negative muons on Helium. The reaction studied is the most fundamental of chemical reactions—that of the isotopic reaction rates of the H +H2 reaction and in particular of the 4Heu +H2 reaction. Comparisons between the experimental results and the predictions of exact quantum rate theory are nearly perfect, one of the very few examples of an exactly-solved problem involving more than two atoms.
 |
| Diagram of the muonic isotopes of hydrogen, from New Scientist magazine. |
Though it may not be obvious, Nature's most fundamental chemical reaction does indeed involve the dance of just three H-atoms, as noted by Prof. Millard Alexander of the Chemistry Dept., Univ. of Maryland, who wrote a "Perspective" on this Science article. One might even think of it as "the quark of the molecular world." It involves a dance of three partners in close proximity changing hands, one hydrogen atom for another. In fact, what is most spectacular, in changing partners from muonium (Mu), the lightest isotope of hydrogen, formed by a positive muon and an electron and which has a mass only 1/9th that of the H atom, to the other extreme, its heaviest isotope, "muonic He" (4Heu), formed by negative muon capture on 4He and which has a mass 4x heavier than the H atom, they covered a range of 36 in isotopic mass, which is totally unprecedented in the field of chemical kinetics and reaction dynamics.
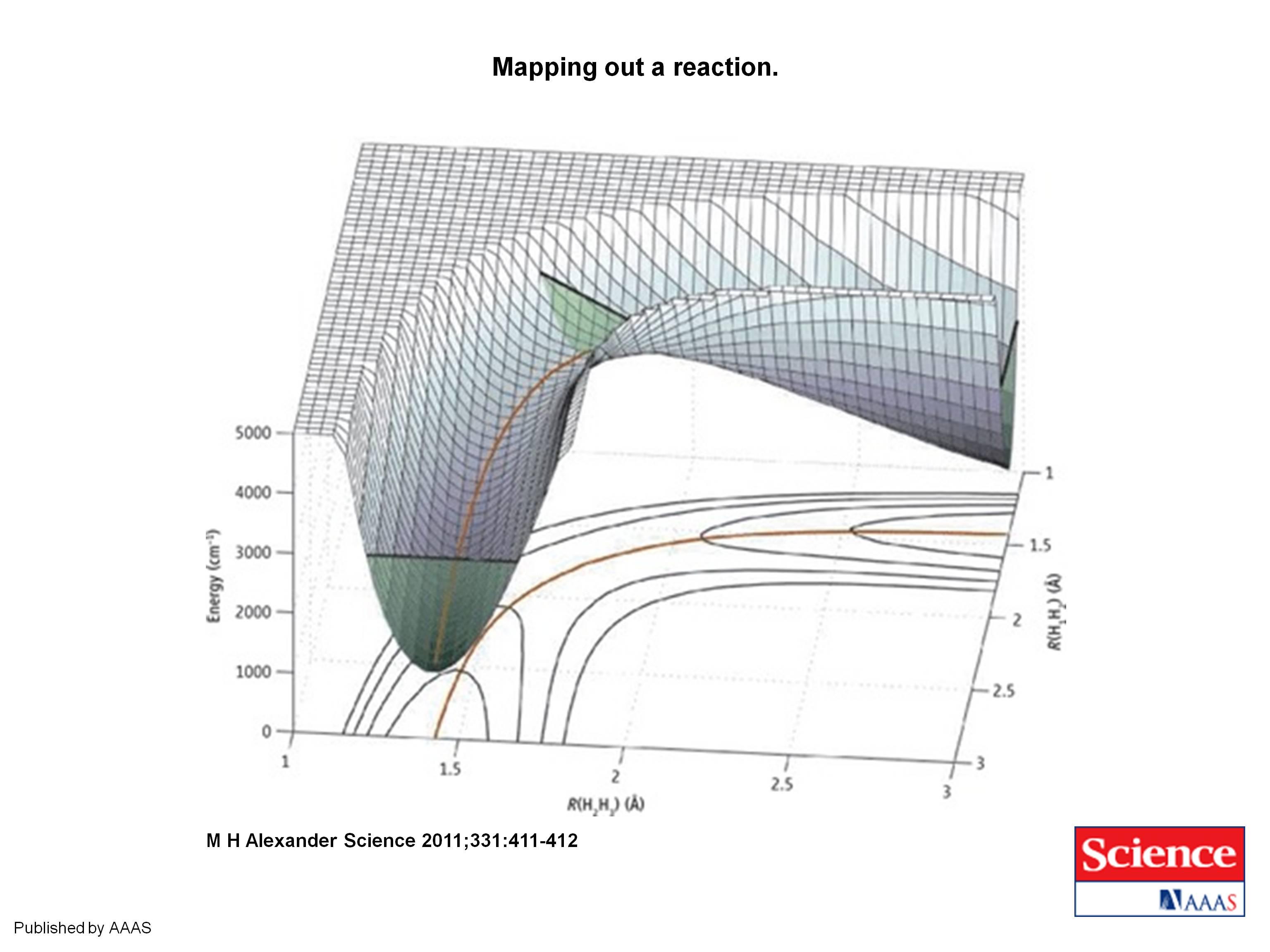 |
| Mapping out a reaction. Surface mesh plot of the potential energy surface for the collinear H1 + H2H3 → H1H2 + H3 reaction as a function of the two bond distances, R, superimposed on a conventional contour plot of the potential energy surface. The minimum-energy reaction path is indicated by the solid orange line. The zero-point energy in the reactant and product arrangements, and at the barrier, is indicated by straight black lines. The three corresponding green slices indicate the range of coordinate space made classically accessible by zero-point energy. (From M. H. Alexander, Science 2011;331:411-412.) |
The reaction rate reported in this paper, the 4Heu +H2 reaction is the first of its kind and is particularly important for this most fundamental of chemical reaction rates since this can now be calculated essentially exactly by quantum mechanics. The present calculations are in fact essentially an exact solution of the Schroedinger equation for the H3 system. In the quantum calculations of reaction rates, the only interaction that can still be truly rigorously treated is the H +H2 reaction, since this reaction involves only three electrons. As the number of electrons in the problem increases the complexity in the quantum calculations increases hugely, always necessitating approximations at some level. One outcome of the present experiment, having now demonstrated just how accurate the theory is on this most fundamental of reaction rates, the H +H2 reaction, is that it gives confidence in similar theoretical methods applied to more complex systems.
-- By D.G. Fleming, with editorial assistance from T.I. Meyer. Don Fleming is the winner of the 2002 CIC John Polanyi Award for Physical Chemistry and Chemical Physics and winner of the 2004 ACS Glen T. Seaborg Award for Nuclear Chemistry. Co-author Jess Brewer is the winner of the 2008 CAP Brockhouse Medal. Both were responsible for seeing the μSR program at TRIUMF developed in the mid 1970s which has evolved into the CMMS facility today.
