Over the last 50 years, technetium-99m has driven unparalleled advances in the development of non-invasive imaging techniques and, in turn, our understanding of disease.
Now, a new cyclotron-based approach to producing this critical diagnostic tool has received Health Canada approval, greenlighting the made-in-Canada technology for national implementation and opening the door to a greener, more reliable way to make technetium-99m.
The approval represents a critical milestone for the TRIUMF-led Cyclomed99 consortium, which spearheaded the innovative research effort. The consortium, including partners BC Cancer, the University of British Columbia (UBC), the Lawson Health Research Institute, and the Centre for Probe Development and Commercialization, is the first in the world to obtain full regulatory approval for cyclotron-based production.

(Image: Andrew Robertson, TRIUMF Research Officer, Therapeutic Isotope Product Development, working in a TRIUMF radiochemistry laboratory)
It also a turning point for the consortium's licensee ARTMS Inc., the TRIUMF spin-off company bringing this technology to market. ARTMS’ technology makes technetium-99m production possible on many of the world’s most common medical cyclotrons, enabling regional production of this critical isotope within local communities.
"Medical isotopes help so many people every day. It's critical to have a stable, multi-faceted supply chain to avoid unexpected disruptions to their availability," said Paul Schaffer, Associate Laboratory Director, Life Sciences at TRIUMF and Associate Professor at UBC's Faculty of Medicine. "The approval of cyclotron-produced technetium-99m by Health Canada is an important milestone for this Canadian innovation that will ultimately deliver direct benefit for Canadian patients."
While the Health Canada approval brings new promise for patients and researchers, it also highlights an important chapter in Canadian innovation, one which saw a focused national research effort produce an effective solution to a global problem.
The path towards cyclotron-produced technetium-99m
By injecting and tracking technetium-99m-labelled tracers, medical professionals and researchers can visualize areas of the body associated with a particular compound. This growing list includes cell signaling molecules, cancer biomarkers, and more.
Today, technetium-99m is the most used of all diagnostic radioisotopes, which collectively enable tens of millions of cardiac tests, cancer scans, and other diagnostic nuclear medical procedures worldwide each year.
However, the process for making technetium-99m has historically come with a significant logistical caveat— with just a 6-hour half-life, the isotope must be used shortly after it is created.
Further, technetium’s precursors continue to be sourced mainly from an ageing fleet of nuclear reactors, once including Canada's National Research Universal (NRU) reactor at Chalk River (the NRU was permanently shut down in 2018).
In 2009, following unplanned disruptions at NRU (which historically provided up to half of the world's technetium-99m via molybdenum-99 generators), the Government of Canada initiated the Non-reactor-based Isotope Supply Contribution Program (NISP) which challenged researchers to find a new way to produce critical medical isotopes—in particular, technetium-99m.
Led by Schaffer and TRIUMF's Dr. Tom Ruth, scientists and engineers from TRIUMF joined partners at BC Cancer, the Centre for Probe Development Commercialization (CPDC), the Lawson Health Research Institute, and the University of British Columbia to launch a national collaboration to answer the NISP call: the 'CycloMed99' consortium.'
A new way to produce technetium-99m
Enabled by TRIUMF's unique accelerator infrastructure and radiochemistry processing facilities, the consortium's technology development progressed quickly. In just over four years, the team successfully developed and validated their systems, initiated clinical trials, and demonstrated the technology’s capacity to serve to serve Canadian communities and lessen reliance on reactors. They created ARTMS Inc. to help commercialize the technology.
"The technology transfer of cyclotron-based technetium-99m with ARTMS, from physics laboratory to market, is a great example of the value of collaborating across scientific research, government, and industry," said Kathryn Hayashi, CEO of TRIUMF Innovations. "The government convened key players around a strategic and concerted funding project; TRIUMF and its CycloMed99 partners contributed the research, development, and supplemental support; and well-timed private sector investment from Vancouver-based venture fund, Quark Venture, all contributed to ARTMS' growth and continued success. With ARTMS, Canada has a leading-edge company – one positioned to revolutionize the market for a high demand medical isotope."
A bright future for Canadians
With technetium-99m now approved for production and clinical use at regional cyclotron facilities in Canada, researchers will have safe and reliable access to one of their most important diagnostic tools. With an on-demand source of technetium-99m, there will be fewer cancelled procedures due to isotope shortages, thereby improving health outcomes for patients.
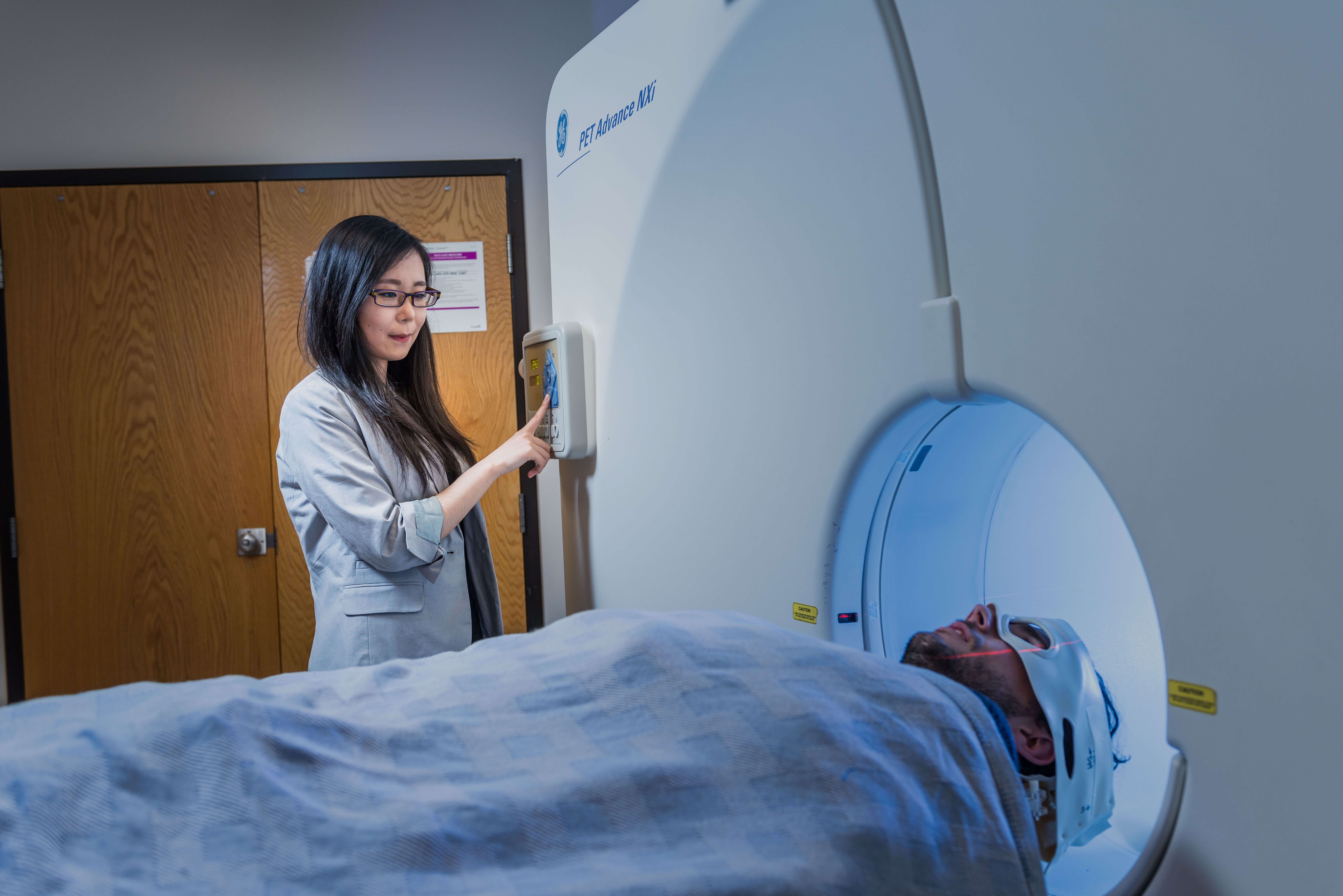
(Image: a patient undergoing a PET scan)
"Cyclotron centres across Canada can produce these isotopes locally and on-demand, and we have shown the path that can be used to achieve regulatory approval," said Francois Bénard, senior executive director of research at BC Cancer, professor of radiology and associate dean of research at UBC's faculty of medicine. "The same approach can be followed at other sites in Canada and internationally. This has been a shared vision of many researchers across the country, and we have to recognize the many collaborators who worked for years to make this announcement possible."
This bright future will first take shape at TRIUMF's Institute for Advanced Medical Isotopes (IAMI), where a state-of-the-art TR-24 medical cyclotron will offer production capacity for the Lower Mainland's technetium-99 needs. In addition, IAMI will serve as a hub for radiopharmaceutical research, providing access to leading-edge facilities and expertise in accelerator technology and isotope science. The Institute will further catalyze the Vancouver region's diverse nuclear medicine sector by convening researchers, students, academic collaborators, not-for-profits, government, and industry partners.
"With support from the Canadian government and our partners, we have developed an effective solution to the medical isotope crisis, one that will improve health outcomes and reaffirm Canada's role as a global leader in isotope production and research. This important work will continue as we leverage our unique infrastructure and expertise with TRIUMF's IAMI,” said Jonathan Bagger, TRIUMF Director. “Today's announcement marks a tremendous achievement for our laboratory, our community, and our collaboration partners.”
