
The focus of the Mount Allison University muon chemistry group, led by Dr. Khashayar Ghandi, is the study of green chemistry, and chemical dynamics using muons as microscopic probes. Green chemistry is the chemistry that designs products and processes which reduce or eliminate the use and generation of hazardous substances to the environment.
The Mount Allison group, with experiments 1131, and 1051 study the reactivity of green chemical reagents as well as reactions in green solvents. Green reagents are environmentally friendly compounds, which will eventually substitute non-environmentally friendly chemical compounds.
One of the group’s contributions is the microscopic study of the free radical reactions. A free radical is an atom or molecule, which has at least one unpaired electron. The unpaired electron could make the molecule very reactive. Therefore, the study of free radicals that are intermediate in chemical reactions is done with special techniques. Muon techniques, such as the ones used at TRIUMF, are among the most informative techniques to study reactive free radicals by providing information on distribution of the unpaired electron.
The more conventional techniques to characterize free radicals are restricted in probing reactive free radicals, in particular in supercritical fluids, which have potential as green solvents. A supercritical fluid is a substance where above a certain temperature there is no distinction between liquid and gas. One of the most promising supercritical fluids in the industry is supercritical CO2, which has the potential to replace harmful chlorinated solvents and is very easy to recycle. We can use muons at TRIUMF to investigate free radical chemistry almost in any environment, including in supercritical CO2.
The other green solvents that Mount Allison group investigated are ionic liquids. Ionic liquids, like table salts, are inherently two-component systems, composed of the same amounts of cations (positively charged ions) and anions (negatively charged ions), but unlike table salts they are liquid at room temperature! They do not have any significant vapour pressure, and this means they do not harm the atmosphere. As well, the contrasting nature of cations and anions, in particular, intimate sites of high electron deficiency and high electron richness, could enable chemistry that is perhaps not possible in normal molecular solvents.
Experiments 1131 and 1051 explored environmentally alternative solvents (ionic liquids and supercritical CO2) to understand the effect of such solvents on the free radical reactions. Free radical chemistry is used significantly in industry (free radical polymerization) to produce Teflon, plastics, and other material. Using muons, the Mount Allison group, have discovered that there is a significant improvement in free radical formation in ionic liquids as compared to the molecular solvents. They also found that supercritical CO2 can be used as a switchable solvent to direct the free radical reactions along different paths. This can help to design an environmentally friendly process that favours a particular product as opposed to a potential by-product.
The facilities at Centre for Molecular and Materials Science (CMMS) at TRIUMF have been crucial in the research of the Mount Allison University Chemistry Department and other researchers worldwide who conduct basic and applied research that contributes to the world’s expertise in how to increase the potential of environmentally friendly chemistry. The researchers on experiment 1131 and 1051 included three Mount Allison students, Philip Cormier, Jean-Michel Lauzon, and Becky Taylor who, in their words, had “the experience of a lifetime,” working on these experiments at TRIUMF.
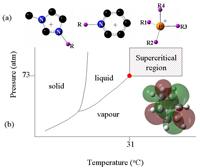
Different phases of CO2, including supercritical CO2 region.
